Therapeutic micro/nano-robots
In an era where science fiction increasingly intersects with reality, our research focuses on the cutting-edge design of micro/nano-robots. These tiny robots are engineered to navigate the human body, delivering therapeutics and capturing pathogens. Their potential to transform medicine is immense. Essential to their functionality is their ability to move autonomously, locate disease sites, identify targets, analyze microenvironmental signals, make decisions, and report outcomes.
A promising approach involves employing multifunctional, stimuli-responsive nanomaterials. These advanced materials aim to boost delivery efficiency and therapeutic effectiveness while reducing off-target side effects. This strategy is key to successfully transitioning nanomedicine from laboratory research to clinical application, addressing the critical challenge of ensuring both efficacy and safety in treatments.
Cell nanoengineering
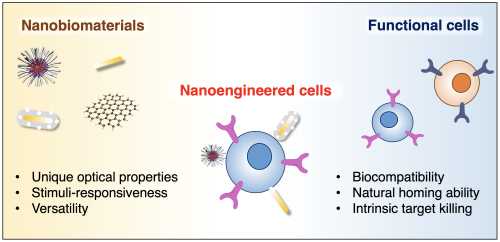
While stimuli-responsive nanoparticles hold promise for smart nanomedicine, their complex design poses translatability challenges. Cells naturally present in our bodies, such as immune and stem cells, already possess remarkable abilities to target and respond to environmental cues, making them excellent candidates for therapeutic applications. However, a significant challenge is monitoring and controlling these therapeutic cells post-administration. By merging nanotechnology with endogenous cells, we can endow these cells with new properties, enabling their tracking and manipulation via imaging and external stimuli. This synergy enhances delivery and effectiveness by navigating biological systems and barriers.
Our lab at UC Davis, the Laboratory for Cell Nanoengineering, is dedicated to developing these nanotechnology-enhanced cells, blending the strengths of cell systems and nanoparticles. Our initial focus is on engineering immune cells with functional nanomaterials for noninvasive, image-guided cancer immunotherapy, and for long-term monitoring and control of immune cell activity. This approach will be expanded to other cell types, like NK cells and stem cells, to refine cell-based therapies further.
Non-invasive and longitudinal imaging
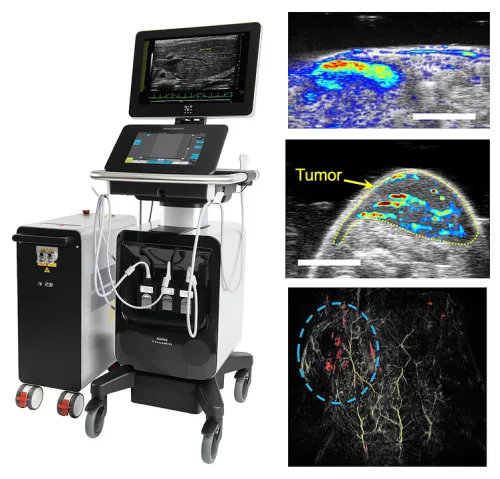
A cornerstone of our research is the non-invasive, longitudinal tracking and functional monitoring of nanoengineered cells using various imaging modalities. Current cell therapy evaluations often rely on invasive techniques that lack real-time insights. We focus on ultrasound-guided and photoacoustic (US/PA) imaging for its non-invasive nature, high spatial resolution, and deep tissue penetration. This imaging method is ideal for clinical use due to its familiarity, portability, cost-effectiveness, and non-ionizing nature. US imaging offers detailed anatomical views, while PA imaging provides functional information through high-contrast, high-resolution signals generated by optical absorption of contrast agents. By employing stimuli-responsive nanoparticles as contrast agents in PA imaging, we can enhance signal detection, allowing for detailed, time-lapse monitoring of cellular and molecular events.
Our mission
The Laboratory for Cell Nanoengineering aims to pioneer in two main areas: 1) developing nanoengineered cells using functional and stimuli-responsive nanomaterials, and 2) tracking and monitoring cell therapies with noninvasive imaging tools, predominantly US/PA imaging. This interdisciplinary approach holds great promise in advancing our understanding and application of various autologous or adoptive cell therapies, marking a significant stride in the field of nanomedicine and bioengineering.
